Services and Free Resources

Outsource Personnel & Person Responsible for Compliance (PRRC)
Services
If you do not want to hire a Regulatory or Quality person permanently due to strategic reasons, CMS offers services that allows you to engage our personnel to work under your company on a temporary or part time basis.
Under EU MDR, a company need to appoint a Person Responsible for Regulatory Compliance (PRRC). This person must have the qualifications and experience to understand CE MDR processes. CMS offers personnel who can fulfil to this need.
Technical Documentations
Services

CMS is able to complete your technical documentations (technical file) for class I(s/m/reusable), IIa, IIb and III by generating clinical evaluation in accordance to MEDDEV 2.7.1, risk analysis in accordance to ISO 14971, software verification & validation in accordance to ISO 62304, cybersecurity protocol, usability validations in accordance to ISO 62366, post market surveillance plan & report, transportation validation.
Free Resources
Click here to see the contents of a technical documentations and also interpretation on the requirements.
Click here to see how to fill up a General Safety and Performance Requirement (GSPR) checklist in accordance to EU MDR.
Click here to see how to draft the EU DOC.
Click here to see frequent mistakes committed when transiting from MDD to MDR.
Click here to see when to implement UDI.
Click here to see common mistakes when generating the Clinical Evaluation Report (CER)

Coordination for testing
Services
We also provide training and coordination services for product testing in accordance to ISO 10993, IEC 60601. In addition our technical team can recommend which test lab to use and which test to perform to fulfil to regulatory requirements.


Quality Management
System (QMS)
Services
CMS can setup your Quality Management System in accordance to ISO 13485, US FDA 21 CFR 820, MDSAP.
Free Resources
Click here to see how to comply to ISO 13485: 2016 Clause 4.2.4 Control of Documents .
Click here to see how to comply to ISO 13485: 2016 Management Review Requirements
Click here to see how to comply to ISO 13485: 2016 Work Environment.
Click here to see how to comply to ISO 13485: 2016 Clause 6.2 Human Resources.
Click here to see how to comply to ISO 13485: 2016 Feedback and Complaint Handling
Click here to see how to comply to QMS for EU MDR and MDSAP.

Audit
Services
CMS can perform audit for your company to ensure your technical documentations and quality management system complies with regulatory requirements.

External Audit
Services
CMS works with external auditors (notify bodies) like DQS, TUV SUD and BSI to help company gain ISO 13485, MDSAP and CE certifications.



Registration and Local Representation
Services
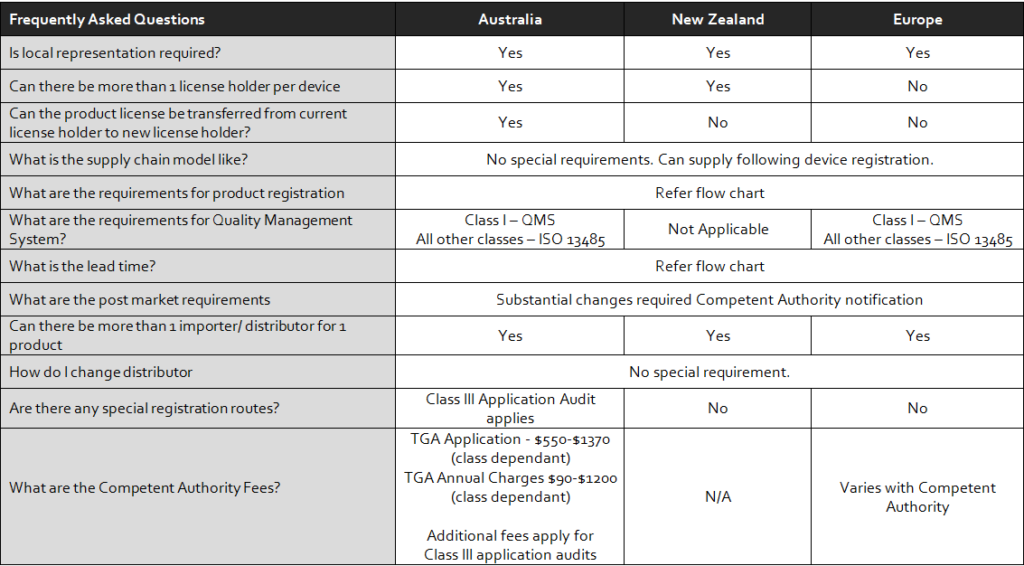
CMS offers medical device registration in Europe and European Authorized Representation services (EC REP)
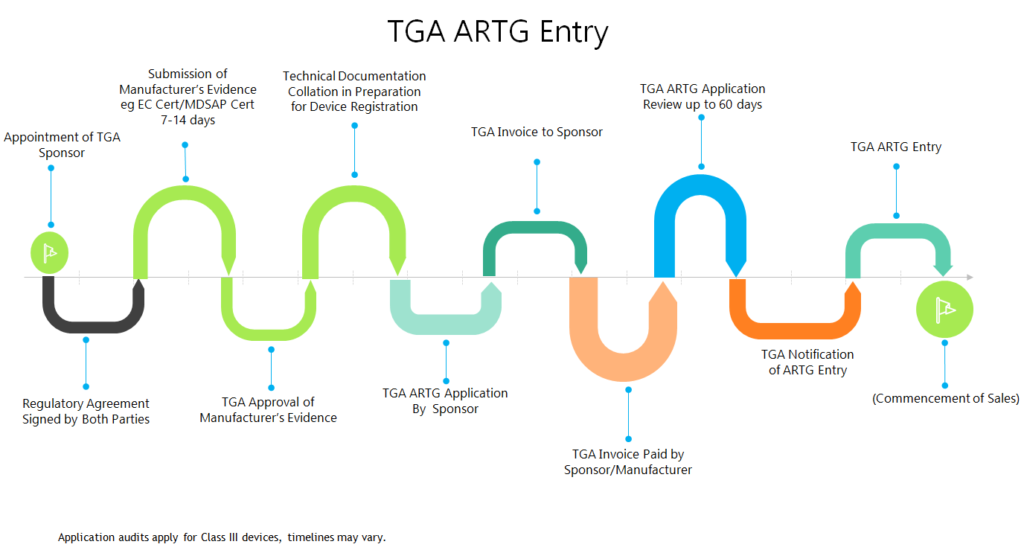
CMS offers medical device registration in Australia with local representation.
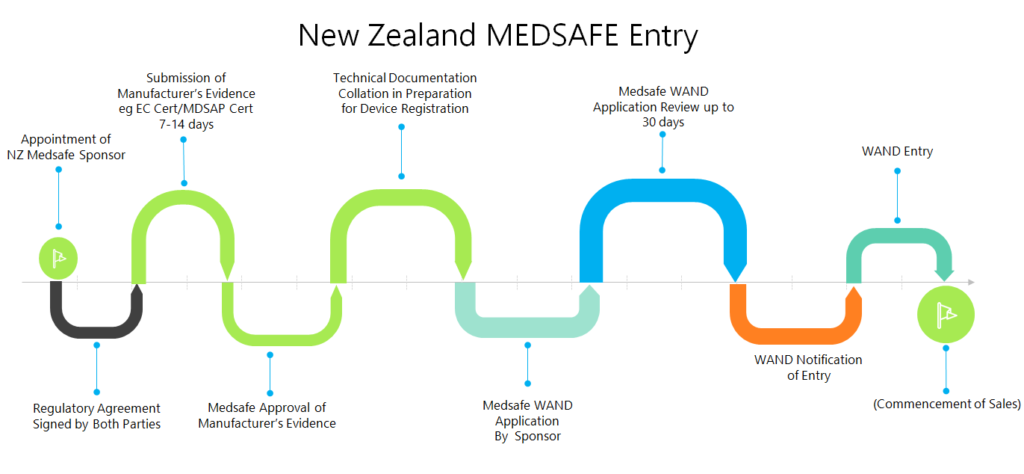
CMS offers medical device registration in New Zealand with local representation.
CMS offers medical device registration in Singapore with local representation.
CMS offers medical device registration in Malaysia with local representation.
CMS offers medical device registration in Mexico with local representation.
And we are still growing!
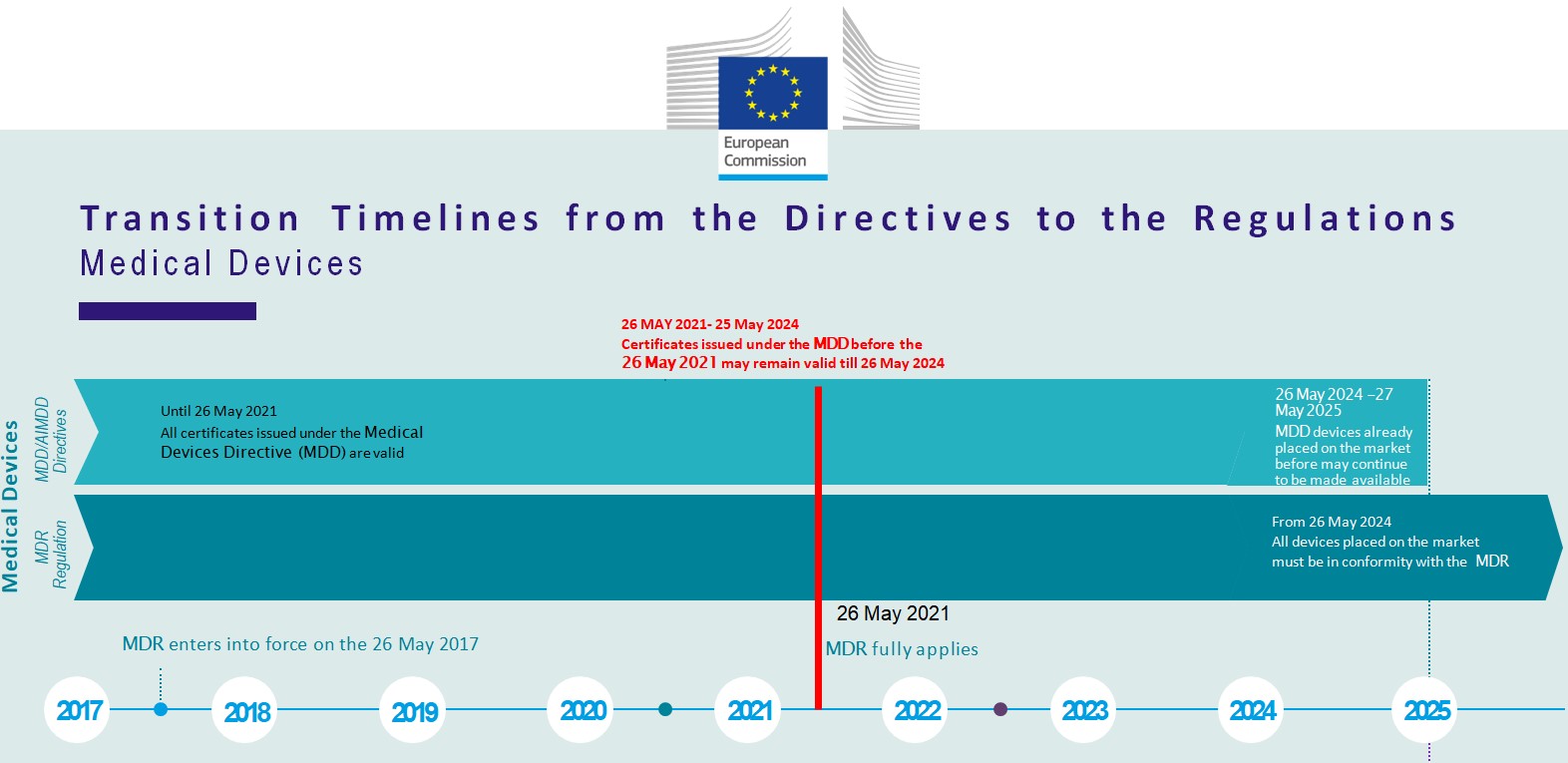
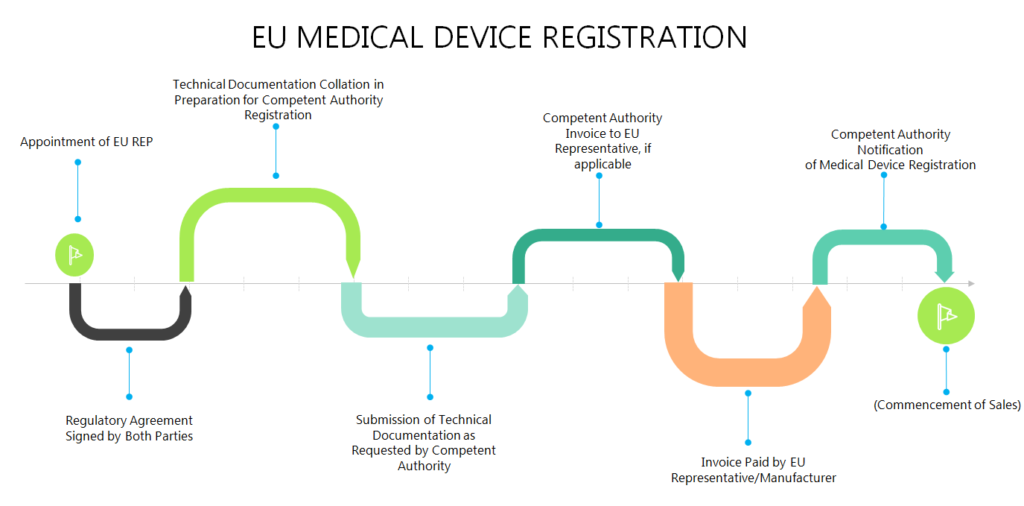
Free Resources
Click here to see Australia registration process.
Click here to see Bangladesh registration process.
Click here to see Brazil Priority Device for Covid 19.
Click here to see Cambodia registration process.
Click here to see CE Class I registration process.
Click here to see CE official website.
Click here to see Europe or EU registration process.
Click here to see how to choose a good EC REP.
Click here to see India registration process.
Click here to see Indonesia registration process.
Click here to see Indonesia authority registration guidance.
Click here to see Japan registration process.
Click here to see Malaysia registration process.
Click here to see Malaysia latest announcements.
Click here to see New Zealand registration process.
Click here to see Pakistan registration process.
Click here to see Philippines published regulations.
Click here to see Russia registration process.
Click here to see Saudi Arabia (KSA) registration process.
Click here to see Singapore registration process.
Click here to see Taiwan registration process.
Click here to see Thailand registration process.
Click here to see US registration process.
Click here to see Vietnam registration process.

Training
Services
CMS provides remote or on-site training on all the topics above.
ISO 13485 – 2 Day Training Course – April, June & Sept 2021
INTRODUCTION :
ISO 13485:2016 Is the basis for a Quality Management System (QMS) implemented by medical device manufacturers. This course explores the requirements of the ISO 13485:2016 Quality Management System standard clause by clause.
DURATION: 2 Day training course
DATE: Q1: 19th -20th April 2021,
Q2: June 2021,
Q3: September 2021.
FORMAT: On site, at CMS SCIDOC or Remote
WHO SHOULD ATTEND?
- Senior Management
- Medical device manufacturers
- Quality Managers
- Regulatory Affairs Managers
- Anyone involved with the implementation of the ISO 13485:2016 standard
LEARNING OBECTIVES:
On completion, you should gain the knowledge and skills to:
- Understand how to use ISO 13485:2016 within your organization.
- Recognize the use of ISO 13485:2016 as the basis of regulatory requirements worldwide.
COURSE BENEFITS:
This course will help you:
- Take the first steps towards ISO 13485:2016 certification
- Understand how you can better meet regulatory requirements.
- Find ways to increase efficiency and cost savings through quality management
- Monitor supply chains to achieve continuous improvement
- Develop safe and effective medical devices
- Help you fulfil your company’s ISO 13485 training requirements.
ISO 13485 Overview – Training Session – 28 April 2021
INTRODUCTION:
ISO 13485:2016 is the basis for a Quality Management System (QMS) implemented by medical device manufacturers.
WHY DO YOU NEED ISO 13485?
- Increase access to more markets worldwide with certification
- Outline how to review and improve processes across your organization
- Increase efficiency, cut costs and monitor supply chain performance
- Demonstrate that you produce safer and more effective medical devices
- Meet regulatory requirements and customer expectations
WHAT MAY GO WRONG IF YOU ARE NOT COMPLIANT:
Nonconformity doesn’t just mean that the organization failed to meet some formal requirements, it can also imply that the processes are not controlled properly, and there is a good chance that the product or service will fail to meet the customer requirements.
Depending on the type of product or service the company delivers, the consequences can be severe to the customer and ultimately to the organization.
DATE: 28 April 2021,
TIME: 10 AM to 1 PM (AEDT)
FORMAT: Remote via Zoom
WHO SHOULD ATTEND?
- Senior Management
- Medical device manufacturers or developers who want to gain better understanding of the quality requirements.
LEARNING OBECTIVES:
- Basic understanding of ISO 13485:2016 requirements.
COURSE BENEFITS:
- Understand how you can better meet regulatory requirements
- Develop safe and effective medical devices
MDR TRAINING MODULE – May & July 2021
INTRODUCTION :
To help medical device manufacturers understand the additional requirements of the MDR, we have developed the MDR Training module. This course will help you to implement the requirements of European Medical Device Regulation to obtain and maintain CE marks for your products.
DURATION: 3 Day training course
DATE: May & July 2021
FORMAT: On site, at CMS SCIDOC or Remote
WHO SHOULD ATTEND?
- Medical device manufacturers
- Quality and Regulatory Affairs professionals
- R&D and Clinical affairs professionals
- Authorized Representatives
- Economic Operators, including importers and distributors
LEARNING OBECTIVES:
This course will help you to understand:
- The MDR’s additional requirements
- Transition arrangements as stipulated within the Regulation
- Requirements for eonomic operators
- Certification requirements
COURSE BENEFITS:
This course will help you:
- Identify the key changes in the transition from the MDD to MDR
- Understand the impact of the regulation for your organization
- Identify the next stages for your organization to meet the requirements
- Implement the requirements to maintain compliance to the regulation
MDSAP TRAINING MODULE – May & July 2021
INTRODUCTION :
The Medical Device Single Audit Program (MDSAP) is developed to allow for a single regulatory audit of a Medical Device manufacturer by a MDSAP recognized auditing organization, to satisfy the needs of multiple regulatory jurisdictions.
DURATION: 3 Day training course
DATE: May & July 2021
FORMAT: On site, at CMS SCIDOC or Remote
WHO SHOULD ATTEND?
- Medical device manufacturers
- Management
- Quality and Regulatory Affairs professionals
- R&D and Engineers
LEARNING OBECTIVES:
This course will help you to understand:
- The fundamentals of MDSAP
- MDSAP audit structure and difference between MDSAP and other QMS audits
- MDSAP internal audit program
- The relationships between ISO 13485 and participating regulatory authority compliance requirements
COURSE BENEFITS:
This course will help you:
- To plan and prepare for an MDSAP audit
- Identify the requirements for your organization for compliance
Website: cmsmedtech.com

