Table of Contents
Medical Device Registration in Singapore: Conclusion to the consultation on Medical Devices Product Classification Guide
1.The Health Sciences Authority (HSA) has concluded the stakeholders’ consultation on the Medical Devices Product Classification Guide on 15 April 2021.
2. This document is intended to assist our stakeholders in determining whether a product is classified as a medical device in Singapore.
3. In summary, the feedback received were supportive of the proposed product classification guide. Majority of the feedback received were to include additional clarifications on certain product types and editorial comments.
4. HSA would like to thank our stakeholders for your feedback on the Medical Devices Product Classification Guide. The feedback received has helped to refine the Medical Devices Product Classification Guide. You may access the finalised Medical Devices Product Classification Guide here.
(Source HSA)
Medical Device Registration in Singapore:
Regulatory Fee Revision for Health Products (Effective 1 July 2022)
The Health Sciences Authority (HSA) regulates health products to ensure that they meet the required standards of quality, safety and efficacy. There are fees in place to help cover the cost of registration, licensing, notification and permit issuance for therapeutic products, medical devices, Chinese proprietary medicines, cosmetic products, oral dental gums and retail pharmacies.
With effect from 1 July 2022, a fee increase averaging 3% will be implemented, with a minimum increase of $1 and capped at $200 per fee item (see List of revised regulatory fees). The last fee increase of the same quantum was in April 2019.
This fee revision is necessary to recover part of the costs for the services rendered to businesses.
We will continue to streamline our processes and deliver value to all our stakeholders. Read about our initiatives here.
(Source HSA)
Medical Device Registration in Singapore:
Product Owner Information in MEDICS (Jan 2022)
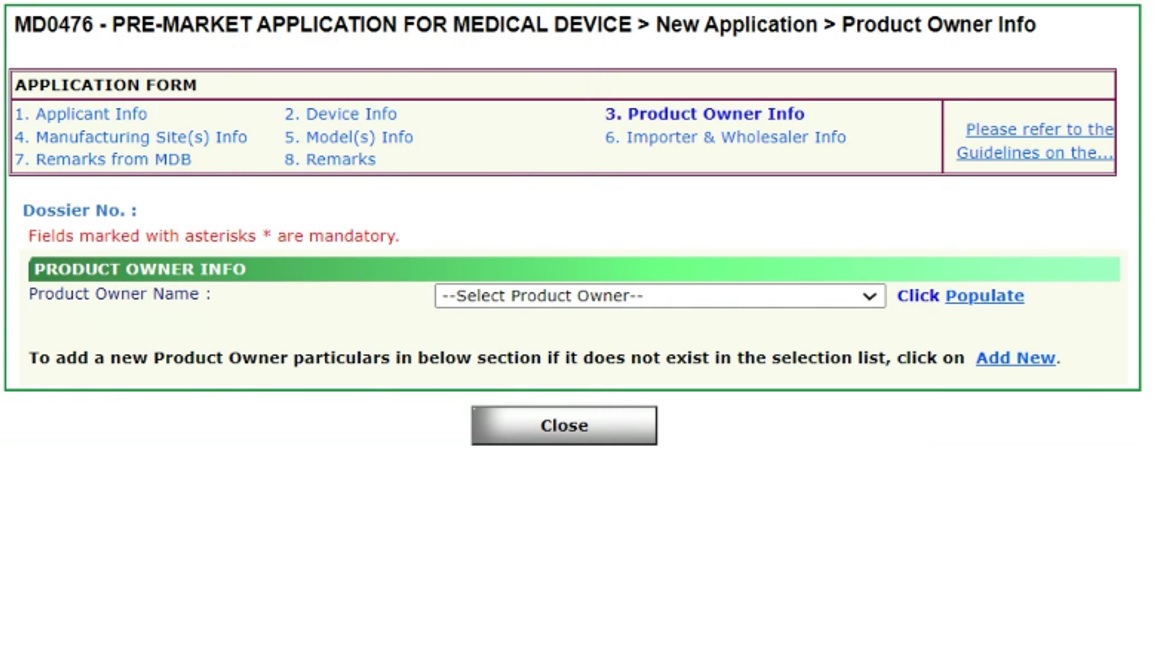
This is with regards to the Product Owner (PO) section in the submission of a new MEDICS Pre-market application. It has come to our attention that applicants have been creating new POs even though the same PO already exists in the PO drop down selection.
We would like to highlight that each new PO entered by applicant will result in a new PO ID being created. This is a potential issue for applicants who wish to submit a new Free Sales Certificate (FSC) and/or Change of Registrant application. Applicants would then be unable to select all SMDR device listings with the same PO name due to different PO IDs being created.
In order to amend the PO ID number to align with the other SMDR listing(s), a Change Notification (CN) submission is required to be submitted by the Registrant. The CN type to submit would be “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)” and there will be no fees chargeable. No other changes will be allowed in this category of CN application submission. Here are the instructions on how to submit this CN:
- Select “Other Change(s) – Applicable only upon receipt of email from HSA, authorising submission under this category” in MEDICS
- Under “Other Change(s)”, select “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)
- Upload the Annex 2 Summary Table of Changes in MEDICS
- Update Product Owner Code under Product Owner Info section in MEDICS for the required SMDR device listing(s)
- Submit a screenshot of the selected Product owner with the Product Owner code for verification
Going forward, we would also like to encourage applicants to first search out for existing POs prior to creating a new PO at the point of a new pre-market application submission.
(Source HSA)
Singapore HSA CEO message 2021.
Please see message here: hsa_ceo_desk_newsletter_2021.pdf (cwp.sg)
Medical Device Registration in Singapore: (Video Explanation)
Medical Device Registration in Singapore
Updated: 18th July 2020
The objective of this post is to explain the Singapore Medical Device regulatory process in layman terms. We would advise that you read through the flow chart explanation below first before embarking on the registration process.
The below flowchart(for medical device registration in Singapore) can be downloaded here.
Singapore important guidance can be access here. These guidance would give more details on the requirements.
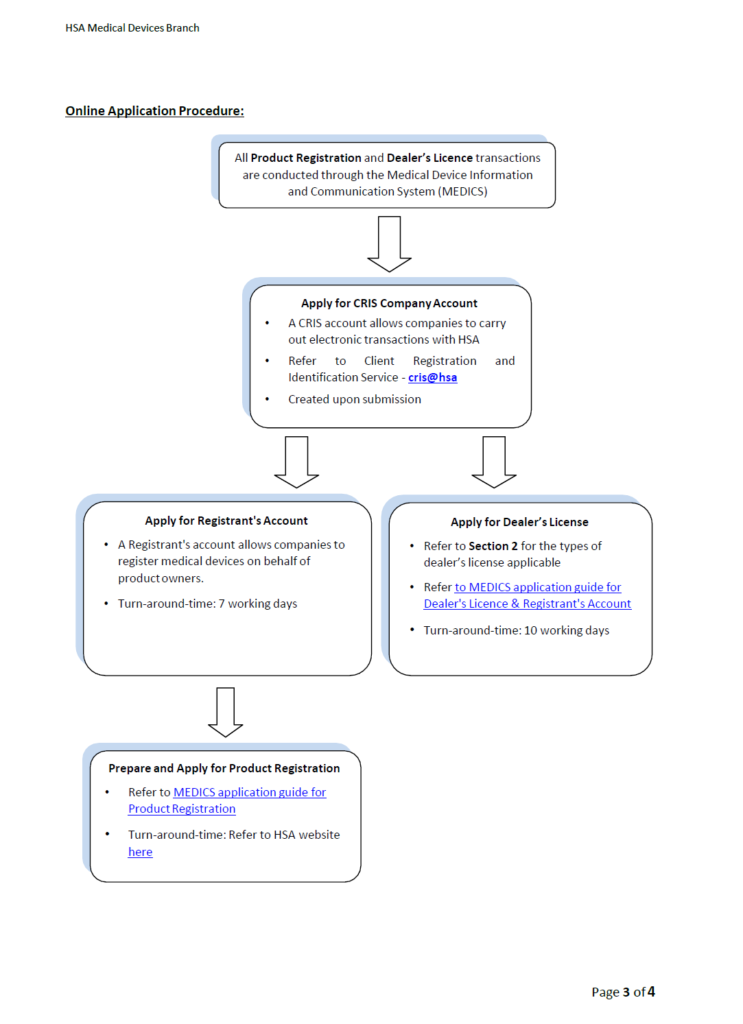
If you are are a Singapore company and you would like to start the registration process please click here. Remember to open your Corpass and CRIS account first and then start the actual registration process in MEDICS.
Please contact CMS here if you need more information.
Frequently ask questions (faq)
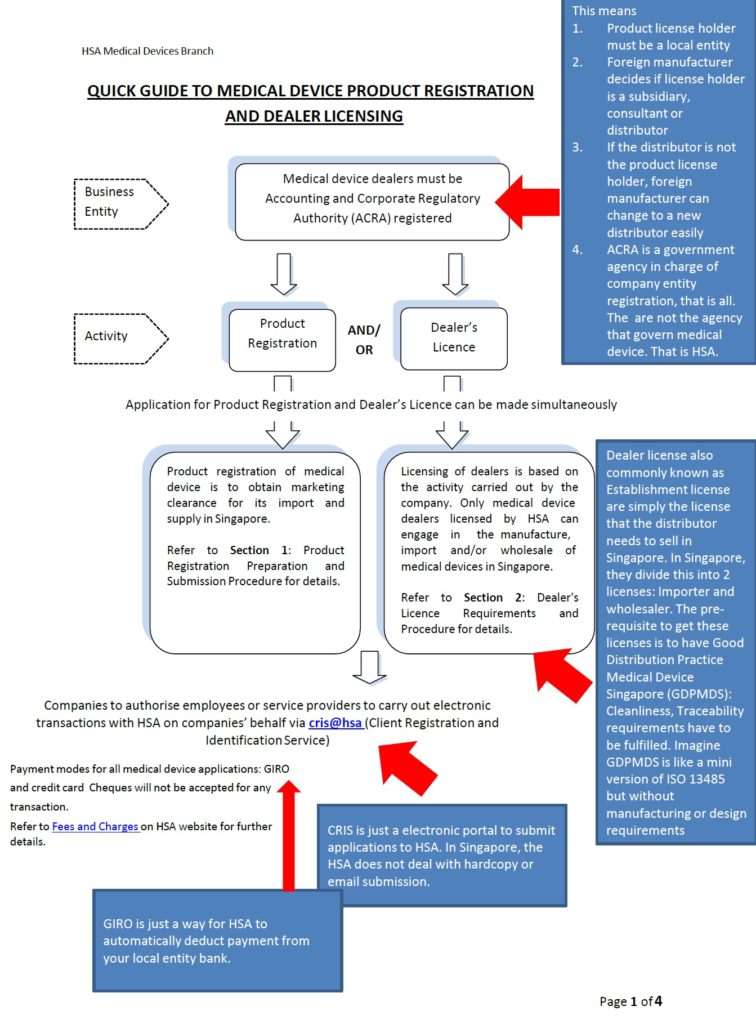
1. Do you need to appoint a local representative to start Medical Device Registration in Singapore?
Class A imported device: Products need to be notified under your legal Singapore importer
Class A local manufactured device: Products need to be notified under the local manufacturing site.
Class B C D imported device: Products need to be registered under your Singapore registrant which can be a subsidiary, distributor or consultant.
Class B C D local manufactured device: Product need to be registered under your Singapore registrant. Usually it is the legal manufacturer in Singapore.
2. Can there be more than 1 product license holder for 1 product
Yes
3. Can the product license be transferred from current license holder to new license holder?
Class A devices: No and it is not necessary, because notification lead time is 0 days after submission.
Class B C D: Yes but you need the agreement from the current license holder
4. What is the supply chain model like?
Class B C D imported device: After product registration is completed. The license holder can appoint the distributor to import and wholesale. No intervention from license holder is needed.
5. What are the document requirements for medical device registration in Singapore.
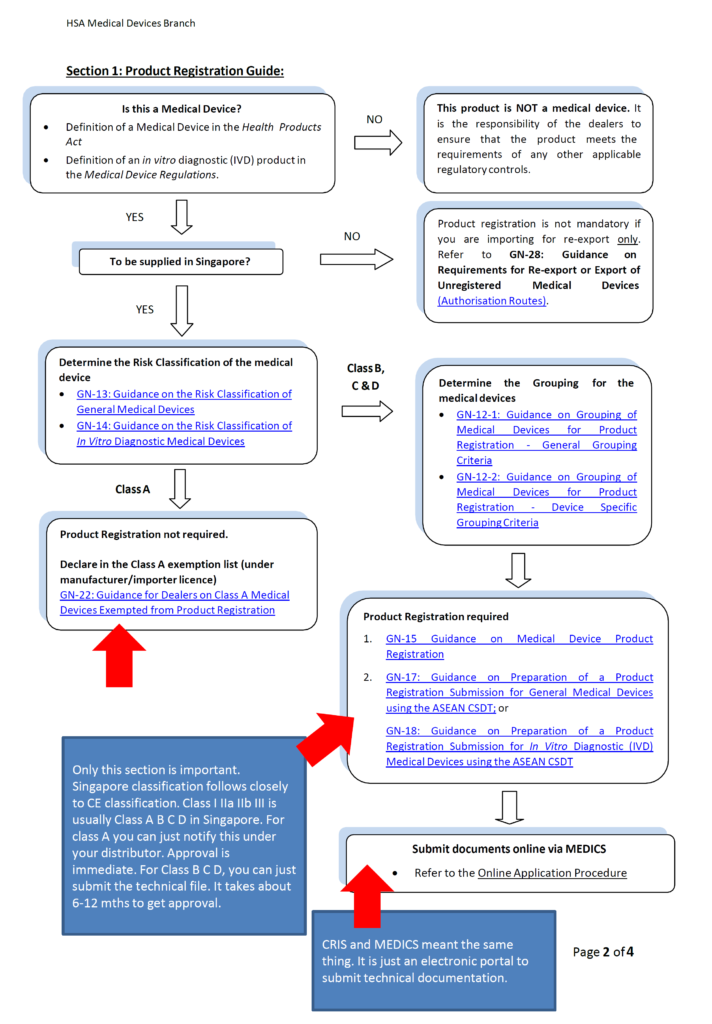
Class A: Product name, product owner and manufacturing site administrative data.
Class B C D: Technical documentations. Please follow our post on technical documentations if you do not know what it consist of. It is basically your technical file. In Singapore, it is call Common Submission Dossier Template (CSDT). It is basically the same thing except Technical file need to be maintained but CSDT is just an application form used at the point of submisson and does not need to be maintained. No Certificate of Free Sale (FSC) or legalization is needed!
6. What are the requirements for Quality Management System?
Class A foreign manufacturing site: None whatsoever!!!
Class A local manufacturing site: ISO 13485 certification or declaration to ISO 13485 process (no cert needed!)
Class B C D manufacturing sites: ISO 13485 certification
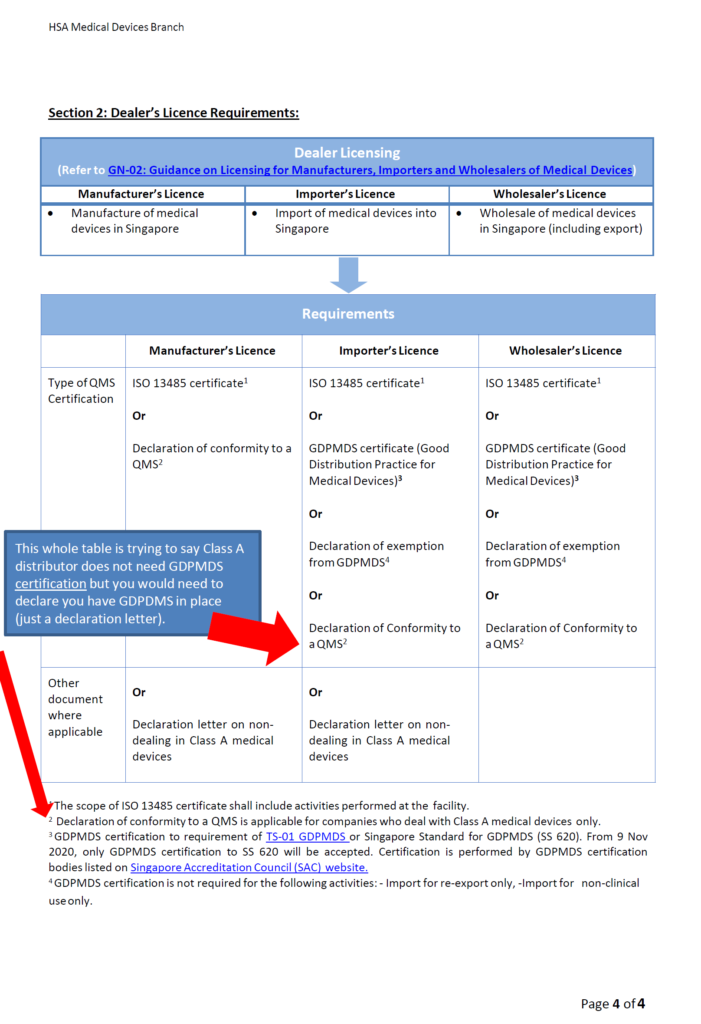
Class A Distributors: Good Distribution Practice Medical Device Singapore (GDPMDS) also known as SS620 certification or declaration to this standard (no cert needed!)
Class B C D Distributors: Good Distribution Practice Medical Device Singapore (GDPMDS) also known as SS620 certification
7. What is the lead time for medical device registration in Singapore?
Class A: 0 days after submission
Class B C D: Usually 6-12 mths and if you meet certain conditions it can be 0 days for class B device and Class C standalone software only.
8. What are the post market requirements?
If there are changes to registered information to Class A, make a new notification.
If there are changes to class B C D devices, make a change notification.
If there is any adverse event or Field Safety Correction Actions (FSCA) that need to be considered in Singapore, you need to inform HSA.
9. Can there be more than 1 importer/ wholesaler for 1 product?
Of course, since you can already have more than one license holder for 1 product.
10. How do I change distributor?
For class A just re-notified under our new importer.
For class B C D medical, just tell you license holder. They can change it within minutes.
11. Is there any special registration routes I should take note of?
Yes, there are for emergency cases, marketing/ training, clinical trials, and for priority devices. Please contact us for more information.
12. What is the cost?
The cost varies between 1-7k SGD or more depending on the classification of the device and approvals (CE, US, Canada, Australia, Japan) you have. Click here for more information on medical device registration in Singapore.
Add Your Heading Text Here
Product Owner Information in MEDICS
Product Owner in MEDICS
This is with regards to the Product Owner (PO) section in the submission of a new MEDICS Pre-market application. It has come to our attention that applicants have been creating new POs even though the same PO already exists in the PO drop down selection.
We would like to highlight that each new PO entered by applicant will result in a new PO ID being created. This is a potential issue for applicants who wish to submit a new Free Sales Certificate (FSC) and/or Change of Registrant application. Applicants would then be unable to select all SMDR device listings with the same PO name due to different PO IDs being created.
In order to amend the PO ID number to align with the other SMDR listing(s), a Change Notification (CN) submission is required to be submitted by the Registrant. The CN type to submit would be “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)” and there will be no fees chargeable. No other changes will be allowed in this category of CN application submission. Here are the instructions on how to submit this CN:
- Select “Other Change(s) – Applicable only upon receipt of email from HSA, authorising submission under this category” in MEDICS
- Under “Other Change(s)”, select “Amendment Changes for correction of typographic errors on SMDR” (Verified by HSA prior to submission)
- Upload the Annex 2 Summary Table of Changes in MEDICS
- Update Product Owner Code under Product Owner Info section in MEDICS for the required SMDR device listing(s)
- Submit a screenshot of the selected Product owner with the Product Owner code for verification
Going forward, we would also like to encourage applicants to first search out for existing POs prior to creating a new PO at the point of a new pre-market application submission.