Table of Contents
Key highlight for New Decree 98/2021
Registration:
- Fast track registration: only request 1 CFS from reference countries
- Add S. Korea and China to reference countries
- Class B medical devices will be registered as per current practice for Class A medical devices. Registration for class B will take about 10-15 days only
Advertising material:
- License holder to be responsible (self check), not required to have approval as current process. LH or agency authorized by LH will post the advertising content on DMEC portal before publishing advertising materials publicly
- Material that only contain name of products but not mentioning product features will not be considered as advertising material.
- Training course with the purpose of instruction for using the device will not be considered as advertising.
Price Declaration:
- Need to declare price of medical devices on DMEC Price Declaration portal before selling the medical device
- Public hospitals are not allowed to buy medical devices that have higher price than declared on the DMEC Price Declaration portal
Risk Classification
- Use AMDD guideline for classification.
- No more certification needed from classification agency
- Applicant for registration will identify the risk classification for medical devices that they want to register.
Implementation of CSDT in Jan 2022
Excerpt from No.: 4882/BYT-TB-CT Hanoi, June 18, 2021
Complying with the provisions of Clause 37, Article 1 of the Government’s Decree No. 169/2018/ND-CP of December 31, 2018, to supplement a number of provisions of the Government’s Decree No. 36/2016/ND-CP of May 15, 2016, on management of medical equipment, which assigns the Ministry of Health to specify how to record in asean general technical dossiers.
In order to gradually strengthen the management and roadmap for regional and world integration in the field of information technology, on the basis of researching and applying the ASEAN Regulation on Medical Equipment (AMDD ) of which Vietnam is a member, on May 15, 2021, the Minister of Health signed Decision No. 2426/QD-BYTon the promulgation of guidelines on how to prepare general technical documents on medical equipment in accordance with ASEANregulations. Decision No. 2426/QD-BYT has been widely posted on the website of the Ministry of Health and the Online Public Service System dmec.moh.gov.vn.
According to the provisions of Clause 3, Article 1 of the Government’s Decree No. 03/2020/ND-CP amending Article 68 of Decree No. 36/2016/ND-CP on management of medical equipment as amended in Decree 169/2018/ND-CP, Asean General TechnicalDocuments (CSDT) are applied from 01/01/2022.
The Ministry of Health shall notify and request the units to urgently study Decision No. 2426/QD-BYT for application. In the course of implementation, if there are problems, request the unit to contact the Ministry of Health (Department of Equipment and Medical Works), Phone: 024.62732272, email: dmec@moh.gov.vn for consideration and guidance on settlement.
One more year of delay?
MoH is drafting a regulation (with high possibility of rolling it out) to delay implementation of decree 36 for class B C D medical devices to Jan 2023 (one more year) and extend implementation of Circular 30 for class B C D till Dec 2022.
Medical Device Registration in Vietnam (Video)
Updated: 20th July 2020 Medical Device Registration in Vietnam
There are currently 2 main regulations (call “Circular”) in place for medical device registration in Vietnam.
New Regulation is call Circular 36. It should be read in conjunction with Decree 169/2018 (which amends Circular 36) It is already mandatory for class A device. It will be mandatory for class B C D device in Jan 2022 (as delayed by Decree 03/2020). However you can start the registration submission now.
The old regulation is call Circular 30. It can be applicable to class B C D products. However products approval issue under circular would only be valid till Dec 2021.
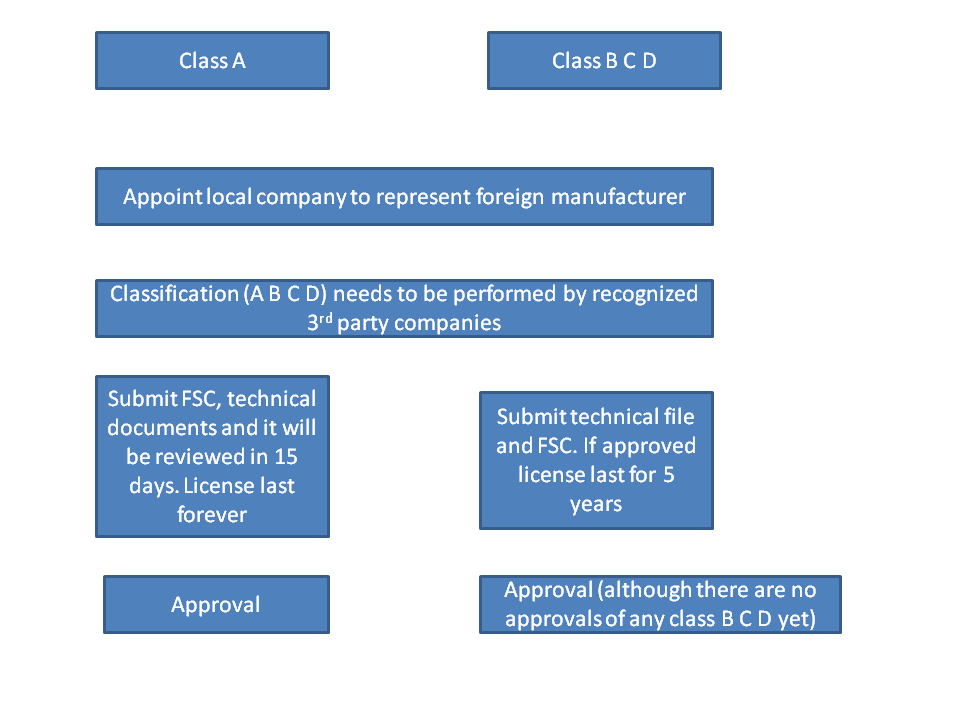
Below is a registration flow diagram for circular 36:
FREQUENTLY ASK QUESTIONS for Medical Device Registration in Vietnam (FAQ)
1. Do you need to appoint a local representative in Vietnam to start the registration process?
Yes
2. Can there be more than 1 product license holder for 1 product
Yes
3. Can the product license be transferred from current license holder to new license holder?
Technically yes, awaiting further guidance from MoH
4. What is the supply chain model like?
After product registration is completed. The license holder can appoint the distributor to import and wholesale. No intervention from license holder is needed.
5. What are the requirements for product registration.
ISO 13485, FSC, Declaration of conformity (can in in accordance to CE), Letter of Authorisation, Letter of Warranty Eligibility (this to declare who is responsible for the repair or exchange and is not applicable for single use device), Technical Summary in accordance to application form, clinical test (application to class C and D invasive device only and can be exempted if there is FSC from one of the 5 jurisdictions namely CE, US, Japan, Australia or Canada), Instruction for use in Vietnamese, labels, official classification from recognised 3rd parties in Vietnam,
6. What are the requirements for Quality Management System?
All manufacturers need ISO 13485
Authorized Representatives need to have legal entity and correct scope in the company license
Importers, Distributors need Purchase and Sale license
7. What is the lead time?
Class A: less than one month
Class B C D: Currently no products have been approved under circular 36 yet.
8. What are the post market requirements?
To be advised when there are clearer guidelines
9. Can there be more than 1 importer/ distributor for 1 product
Yes.
10. How do I change distributor?
Just tell you license holder. They will issue new Letter of Authorization (LOA) to your distributor.
11. Is there any special registration routes I should take note of?
To be advised when there are clearer guidelines
12. What is the cost?
Classification is less than 100 USD
Authority fee is around 100 USD depending on classification