Table of Contents
Medical Device Registration in Malaysia
ANNOUNCEMENT TO PUBLIC: INTRODUCING MEDC@ST 2.0 PLUS IMPROVEMENTS SYSTEM
MedC@st 2.0 Plus System is back with advanced features that facilitate the applicants to experience better.
The new features are including with: –
i. New Change Notification module, will allow (a) Combination Category 2 and Category 3 in an application for SINGLE submission ID number and (b) Combination Category 2 and Category 3 in an application for MULTIPLE submission ID numbers (for a same risk-based classification).
ii. Various selections of notification types developed for Device Study sub – module (under Clinical Research Module) AND submit online notification starting from new notification until completion of study (including Adverse Event Reporting and Scheduled Progress Report).
iii. For the sub-module of Clinical Research Use (under Clinical Research Module) new online form has been developed as well as a more structured Subsequent Notification process that allow notification to be made repeatedly. The new additional feature of the system allows the applicant to download and print the notification letter directly from their MeDC@St account (this is also a new feature embedded in the Device Study sub-module) New features, selection of Medical Device Category under Demonstration for Marketing Notification application.
iv. Additional of payment method which is credit card payment method
v. Auto generated notification letter/ Change notification letter can be printed by the applicant.
No more single AR for Malaysia
PBPP would like to inform that PBPP Circular Letter No. 1/2014 for Establishments Carrying Out the Role as an Authorized Representative (AR) and Establishments Carrying Out Various Activities has been canceled effective 17 June 2021. The implementation to allow the registration of a medical device by multiple authorized representatives (Multiple AR) came into effect on 17 June 2021. However, the implementation of only one license for each role (role)/activity as also stated in the Circular Letter is postponed for now and will be implemented on a date to be determined later. chief executive Medical Device Authority 30 July 2021
Advertisement registration process
Interested to know more on how to register advertisement in Malaysia (applicable to advertisements that are targeted at public and not healthcare professional)
Please click the link below MDA/GL/04 to learn more!
Advertisement registration timeline
MDA has published Medical Device (Advertising) Regulations 2019 P.U. (A) on 15 November 2019. It is supposed to come into operation on 1 July 2020. A circular letter was published that listed the transition period is from 1 July 2020 to 31 Dec 2021 (18 months).
During the latest MDA seminar, the tentative schedule is as follows:
Jan 2021-Mar 2021
Awareness time to disseminate the information of the Medical Device Advertisement
Apr 2021-July 2021
Pilot implementation
July 2021-July 2022
Administrative transition phase. During this stage, there is no enforcement action but there will be more advice/ consultation from the Authority to the industry if they found any non-compliance pertaining to the advertisement
Aug 2022
Full enforcement.
Please contact CMS if you need more information.
Medical Face Mask and Respirator

A new guidance document from MDA has been published. It specifies the requirements for medical face masks and respirators that are regulated under the Medical Device Act (Act 737). This document is applicable to establishments, healthcare facilities, and public dealing with medical face
mask and respirators.
See the requirements here.
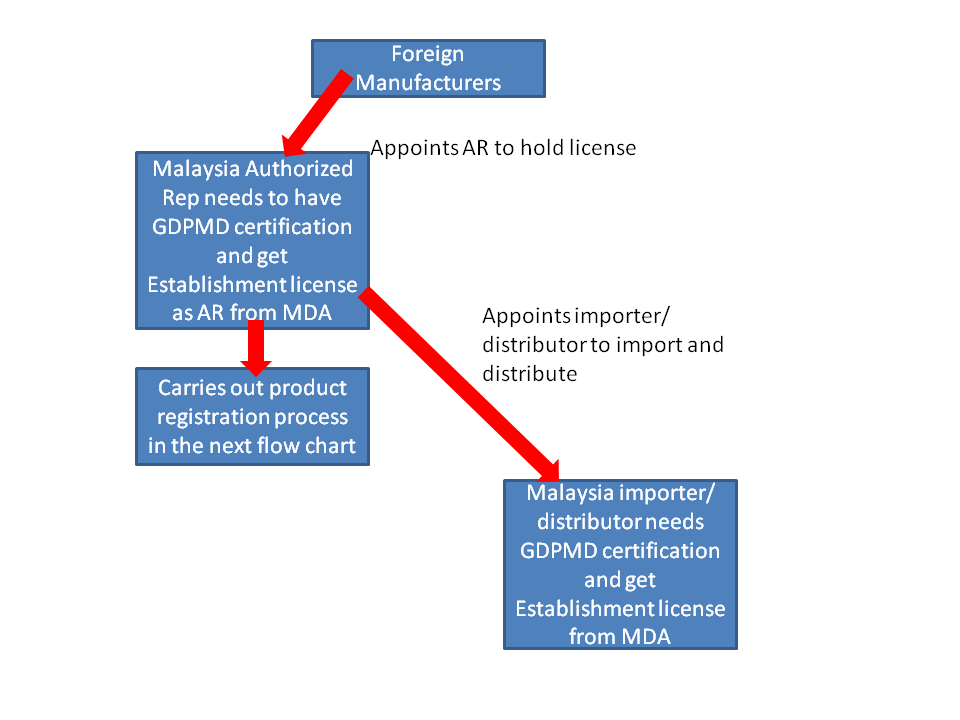
There is no flow chart for Medical Device Registration in Malaysia in the authority website. The objective of this post is to explain the Malaysia Medical Device regulatory process in layman terms. We would advise that you read through the flow chart explanation below first before embarking on the registration process.
Malaysia important guidance can be access here. Malaysia circular letters can be access here. Malaysia legislation can be access here. It is required that you read all 3 sources of information to get the full picture.
If you are are a Malaysia company and you would like to start the registration process please click here. Remember to setup your Medcast account first. To qualify as Authorized Representative you need to have Good Distribution Practice Medical Device (GDPMD) certification.
Please contact CMS here if you need more information on Medical Device Registration in Malaysia.
Flow chart for Medical Device Registration in Malaysia
FREQUENTLY ASK QUESTIONS (FAQ)
1. Do you need to appoint a local representative in Malaysia to start the registration process?
Yes
2. Can there be more than 1 product license holder for 1 product
No
3. Can the product license be transferred from current license holder to new license holder?
Yes, but you need the agreement from the current license holder.
4. What is the supply chain model like?
After product registration is completed. The license holder can appoint the distributor to import and wholesale. No intervention from license holder is needed.
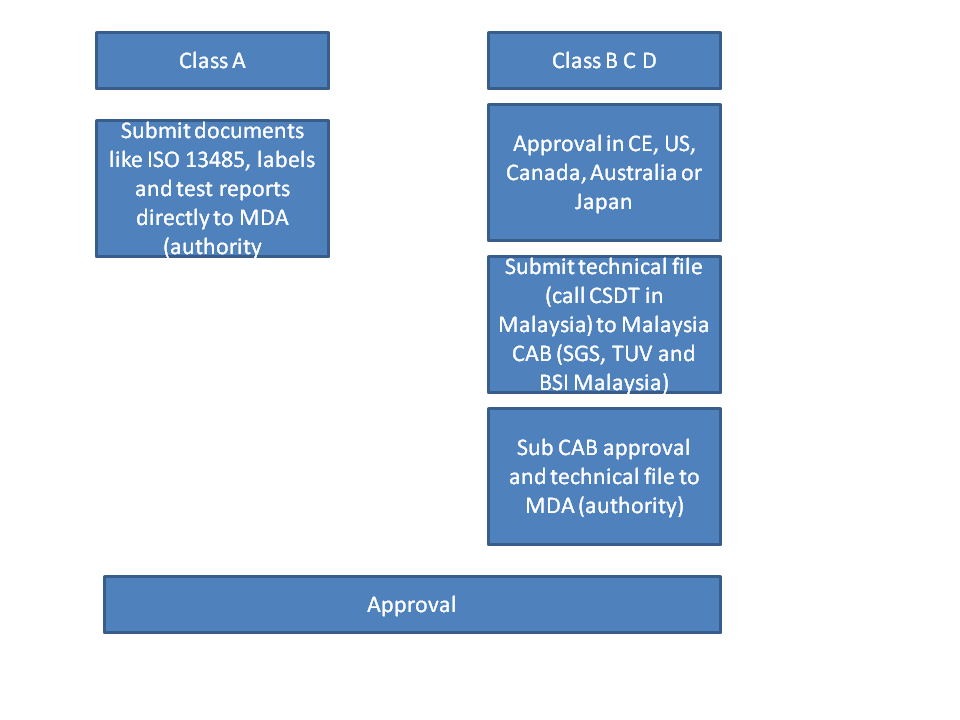
5. What are the requirements for product registration.
Class A: ISO 13485 certification from legal manufacturer and manufacturing sites, test reports and labels.
Class B C D: Technical documentations. Please follow our post on technical documentations if you do not know what it consist of. It is basically your technical file. In Malaysia, it is call Common Submission Dossier Template (CSDT). It is basically the same thing except Technical file need to be maintained but CSDT is just an application form used at the point of submission and does not need to be maintained. No Certificate of Free Sale (FSC) or legalization is needed! But your product needs to be approved by one of the agency (US, Canada, CE, Japan, Australia) otherwise it would be very costly and complicated to get approval in Malaysia (NOT RECOMMENDED).
6. What are the requirements for Quality Management System?
All manufacturers need ISO 13485
Authorized Representatives, Importers, Distributors need Good Distribution Practice Medical Device (GDPMD) certifications.
7. What is the lead time?
Class A: 2-4 mths
Class B C D: Usually 4-7 mths
8. What are the post market requirements?
If there are changes to devices, make a change notification.
If there is any adverse event or Field Safety Correction Actions (FSCA) that need to be considered in Malaysia you need to inform MDA. Currently if there are any adverse events happening outside Malaysia, you would also need to inform MDA (but this will change soon).
9. Can there be more than 1 importer/ distributor for 1 product
Yes. They will be appointed by that one AR.
10. How do I change distributor?
Just tell you license holder. They will issue new Letter of Authorization (LOA) to your distributor and distributor is supposed to update MDA on receiving this new LOA.
11. Is there any special registration routes I should take note of?
Yes, there are for emergency cases, marketing/ training, and clinical trials. Please contact us for more information.
12. What is the cost?
The cost varies between 100 to 3750 RM. Click here for more information. Scroll down to the last few pages to see the cost.
13. What are the post market requirements
You need to make change notification if you made changes to the product. Category 2 (affects safety and performance) change are like major changes and can only be implemented after MDA approved the application. Category 3 which are minor changes (that does not affect safety and performance) change can be implemented immediately upon submission to MDA. You also need do incident reporting whether the incident happen in Malaysia or outside Malaysia (if there is no action to follow up with that incident).



