
Table of Contents
Medical Device Registration in Mexico
Medical Device Regulation change effective 1st June 2021
As part of our goal to keep you informed about the latest news regarding the
regulatory process for medical devices in Mexico, we want to share with you our update about the new regulations in Mexico.
Today it was published in the Official Diary of Mexico a major change to the
Reglamento de Insumos para la Salud, effective TOMORROW June 1st; which includes Medical devices regulation, the main and more impacting changes are the following:
1. No more English-Spanish translations: Effective immediately, Cofepris will be receiving dossiers in English besides Spanish, which means that no more translations English Spanish will be required: technical translations nor Certified translations.
Therefore, the translations that have been already approved by you (if any) will be continued and charged as usual, and they will be submitted with the dossier the old way, for dossiers that are still under review without translations, they will be submitted in English, we will continue the process as usual just skipping translations.
Please note that this is only applicable to English translations, any other language will continue with translations.
2. Change in renewal timing: Once a registration is approved it will be approved for 5 years, and then we will have 270 days before it expires to submit renewal for registration. Before the timeline was 150 days before expiration.
Once the registration is renewed, it will be no need to submit more renewals, unless the product has safety issues and Cofepris explicitly requests more renewals for that specific registration.
3. Less requirements for renewals: We will not need to include labels nor
Certificates of Analysis in the renewal submission anymore.
4. Undefined validity for registrations: For registrations that has been already
renewed, the validity will be undefined, which means no expiry date, so no more renewals will be needed unless there are safety issues with the product.
Please allow us a few weeks to update our databases with this new information and we will come back to you with the new proposal for renewal. On the other hand, there are some gaps in the regulation, and meanwhile they are filled we will continue to operate as usual.
It is important to comment that TECHNOVIGILANCE IS NOT AFFECTED, so please provide your TV information if it has already been requested to you.
5. Third parties: Cofepris is removing from the regulation the possibility of using third parties for fast track, they will not be applicable anymore.
There are some other modifications in the form that the submissions are organized and other changes that are not so generally relevant, we will inform you about these changes just in the case they apply to your specific products or the terms of our services.
On the other hand, Cofepris will need to make adjustments to other current
regulations in order to be compliant with these changes, the most relevant will be the Technovigilance norm. They have established a timeline of 90 days to inform these changes.
We will keep you informed about them as soon as they become official, and the implications
We expect that these changes are positive for you.
Do not hesitate to come back to us in case of any doubts
What is considered a medical device in Mexico?
Medical device:
▪ Medical equipment
▪ Prosthesis and functional aids
▪ Diagnostic agents
▪ Surgical and healing supplies (medical materials)
▪ Hygiene products
Medical devices require registration (Marketing authorization) and it is valid for 5 years which can be renewed.
Some situation requires importation permit for the medical device after registration for each importation.
What do you need to be a registration holder in Mexico?
1. Legally established company in Mexico
2. Legal representative with permanent residence in Mexico
3. Operational warehouse with quality system (SOPs) – notified to MoH
4. Sanitary responsibility (Pharmacist, biomedical engineer or chemist) – notified to MoH
5. Techno-vigilance unit with a techno-vigilance responsibility – notified to MoH
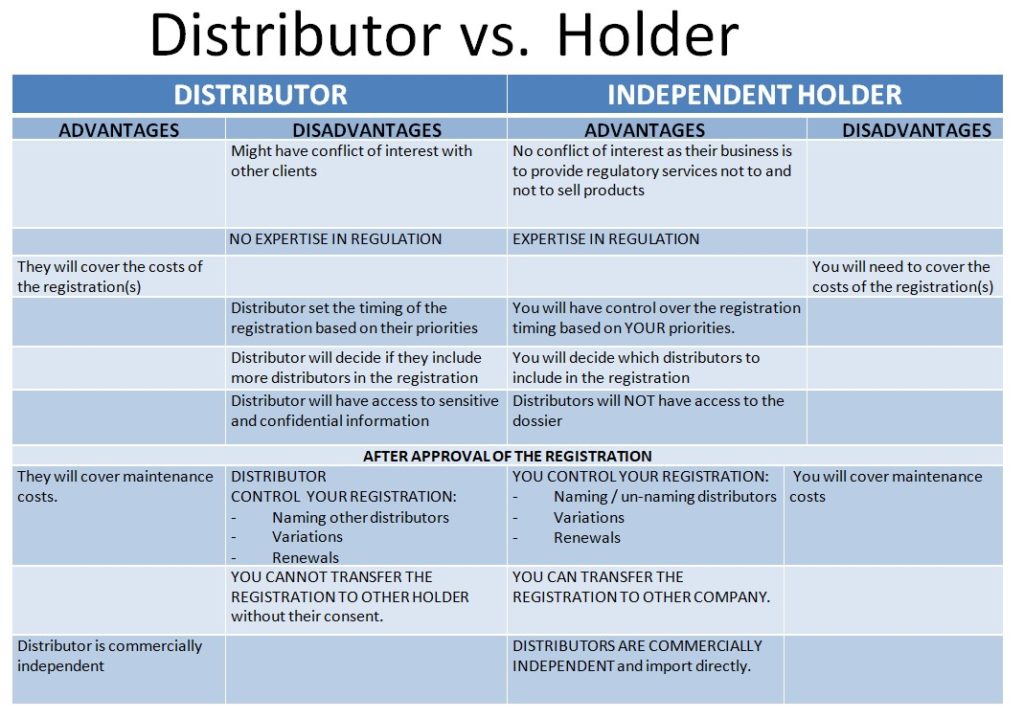
Options?
1. Establish your own company in Mexico.
Everything must be set up and notified to MoH before the submission of registration application.
2. Have another company to be the registration holder for you.
a) Your distributor
b) An independent holder company
Grouping general criteria for Medical Device Registration in Mexico
Same manufacturer
Same class (according to Mexican Criteria)
Same intended use and technology
Same materials – formulation (active ingredients)
All items that can be sold separately must be registered separately (Example: consumables)
Final decision to be decided by MoH only
Classification general criteria for Medical Device Registration in Mexico
Classes:
▪ IA – low risk
▪ I – Mainly external products – non sterile
▪ II – Sterile or invasive < 30 days
▪ III – Invasive > 30 days & implants
Classification/grouping is according to Mexican criteria and ruling despite the classification in the country of origin.
Medical Device Registration in Mexico submission requirements
1.Free sale certificate (FSC) issued by the Health Authority from the Country of Origin or clinical trials performed in Mexico. Legalized original.
2. GMP certificate or ISO 13485 for every facility involved in the process (Manufacturing, sterilization, packaging, etc.) Legalized original
3. Representation letters or Power of attorney naming holder and distributors (issued by manufacturer) Legalized original.
4. Technical dossier: must document every step from materials, manufacturing, finished product, demonstrating quality, safety and efficacy, evidence is required.
5. Original Certificate of Analysis or Conformance.
6. Everything must be submitted in Spanish and organized acc. To MoH Criteria.
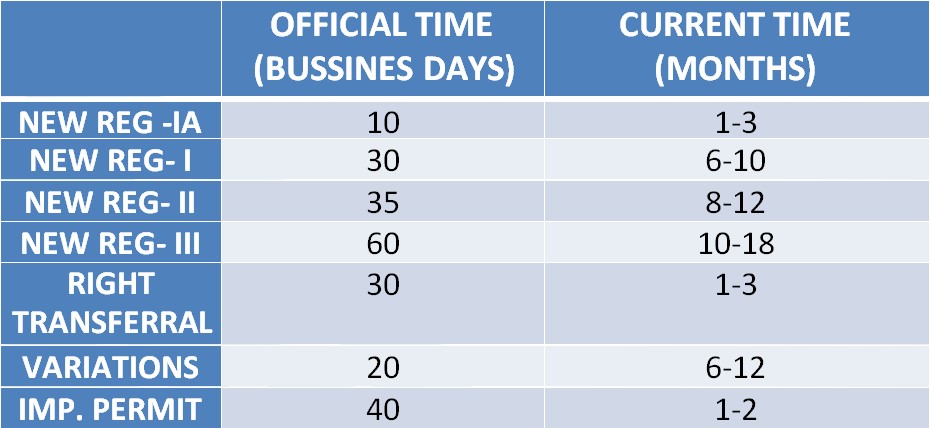
Registration Timing for Medical Device Registration in Mexico
Applicable regulations Medical Device Registration in Mexico
General Health Law (Ley General de Salud -LGS)
Regulations for health supplies (Pharmaceuticals and Medical devices) (Reglamento de Insumos para la Salud – RIS)
FEUM (Mexican Pharmacopeia)– Supplement for warehouses
Applicable norms:
▪ NOM-241 SSA-2012: GMPs for medical devices (in revision)
▪ NOM-137 SSA1-2008: Labeling for medical devices
▪ NOM- 240 SSA-2013: Technovigilance (in revision)
Biocompatibility testing regulation corresponds to the international
Post-marketing obligations*
Maintain a safety surveillance system (techno vigilance) notifying to MoH for any adverse reaction and generating a PSUR every 5 years
Maintaining a quality system, allowing tracking of every batch/serial number sold, to be able to recall product if needed.
Label all products as required by labeling norm.
Get MoH permission for advertising prior to diffusion: public or professional advertising (based on
registration)
Renew the registration every 5 years (approx. 1 year before expiration date).
* Besides warehouse quality obligations
Qualifications of our Mexican consultants
Solid background in regulatory positions within the pharmaceutical industry: 10 years in local and multinational companies (US and European)
Started in 2001 as free lance consultants until we constituted a company in 2013
Our core is regulatory affairs consulting and regulatory intelligence from the start up of the company to registration to the post-marketing support.
In 2013 we added to our portfolio for pharmacovigilance and techno-vigilance services.
We know what your company needs, and how to help you.
Our staff is composed for professionals with degrees related to Health Areas, highly trained and bilingual.



