
Table of Contents
Medical Device Registration in Australia
Regulation of Personal Protective Equipment and COVID-19
In response to COVID-19, there is increasing interest in understanding the regulation of Personal Protective Equipment (PPE) that might be used for therapeutic purposes including face masks, gowns and gloves. The following guidance from TGA in this link is designed to provide:
An overview of how these products are regulated
Information for manufacturers of PPE to help meet regulatory obligations
Information about including a medical device in the Australian Register of Therapeutic Goods (ARTG)
Information for consumers of PPE, including healthcare professionals.
CMS has help several manufacturers registered medical device PPE in Australia. Contact CMS at info@cmsmedtech.com for more information.
Software Regulatory Changes
https://www.tga.gov.au/sites/default/files/regulatory-changes-software-based-medical-devices.pdf
Timeframe for changes to the new classification rules for software-based medical devices are as follows:
To be eligible to continue to supply your eligible medical devices under the transition arrangements, you must:
- Notify the TGA that you have an eligible inclusion by whichever is the later date: – Before 25 August 2021
Note
If you do not notify the TGA of your medical devices before 25 August 2021, you must cease supplying the medical device from 25 August 2021.
If you notify the TGA you have a device that must transition to a higher classification before 25 August 2021, you must submit an application for inclusion before 1 November 2024.
If you do not submit your application by 1 November 2024, you must cease supply on or before this date and cancel your inclusion.
If your application for inclusion is rejected you are no longer eligible for the transition arrangements and must cease supplying your device immediately and consider cancelling your inclusion.
Australian TGA Changes for Software based Medical Devices
Last Day 25 FEB 2021, TGA to be notified for current ARTG entries by 25 AUG 2021.
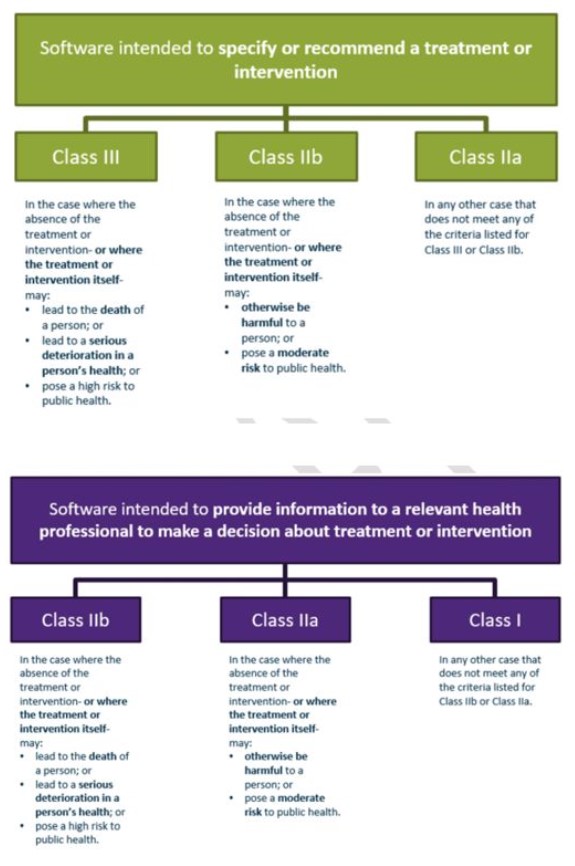
The TGA has proposed new classification rules for software-based medical devices, including smart phone apps. This means that classification rules are now based on risk to individual or public health rather than the intended purpose.
In summary, very few software based devices will be class I.
Examples of new classes as per below, Transition periods apply for those currently ARTG entered.
SPEAK TO US TODAY for further information:
Is your software regulated in Australia?

This guidance summarises the regulatory approach of the Therapeutic Goods Administration (TGA) for software based medical devices.
The TGA regulates medical devices in Australia, including software and mobile apps that meet the definition of a medical device. If a software product is a medical device, it must be included in the Australian Register of Therapeutic Goods (ARTG), unless it is exempt, before it can be legally supplied in Australia.
TGA post-market review on face mask

The TGA is currently undertaking a post-market review of face masks included in the Australian Register of Therapeutic Goods (ARTG). The TGA is aware there are many different standards available to manufacturers; and we are familiar with differences between these standards. The TGA has also identified common areas of non-compliance against claimed standards throughout this review process. This Guidance intends to assist manufacturers in choosing appropriate standards and to set out the TGA’s expectations for performance testing of respirators, surgical respirators and medical/surgical facemasks, before inclusion on the ARTG.
The information provided may be useful to manufacturers and sponsors, as well as health care facilities and health care personnel. This information is not exhaustive and not designed to be a checklist for compliance with the Essential Principles. This guidance will be updated periodically to provide further clarity for both manufacturers and sponsors. Link here.
Medical Device registration in Australia (General document requirements)
Click here to visit Australia TGA (Authority) website.
Click here to see list of medical devices approved in Australia.
Click here to contact us for more information and our service on medical device registration in Australia.
Requirements for medical device registration in Australia
Technical File Summary
completed TGA DOCs for each device
Biocomp evaluation
Clinical evaluation report
Risk management report (plan, analysis and report)
Labels and IFU (if any IFU) – please note that it should include TGA sponsor details as per agreement
Test reports
Contents of Australia DOC for medical device registration in Australia
MANUFACTURER’S DECLARATION OF CONFORMITY
AUSTRALIAN THERAPEUTIC GOODS (MEDICAL DEVICES) REGULATIONS 2002 DECLARATION OF CONFORMITY PROCEDURES
This is a declaration of conformity made under clause 6.6 of Schedule 3 to the Therapeutic Goods (Medical Devices) Regulations 2002.
Manufacturer’s name: Person responsible for manufacturing the device
Business address: Address of the manufacturer
Medical device(s): The unique product identifier (for example the product name or model number)
OR see attached schedule for multiple products
Classification: Class of device (Class IIa, Class I Sterile, Class I Measurement, or Class I) OR Class 1 & Class 2 IVD
OR see attached schedule for the class of multiple products
GMDN code and term: GMDN code and preferred term
OR see attached schedule for the GMDN code and term of multiple products
Scope of application: Products to which the declaration of conformity procedures applies this may include all OR specific or ranges of batches, lots or serial numbers, OR times of manufacture OR see attached schedule for multiple batches, lots or serial numbers
For sterile Class IIa & Class I sterile devices
Each kind of medical device to which the declaration of conformity procedures applies, the production quality assurance procedures have also been applied. Each kind of medical device to which the technical documentation applies complies with the applicable provisions of the essential principles, the classification rules, and these procedures.
For non-sterile Class IIa, and non sterile Class I devices with a measuring function
Each kind of medical device to which the declaration of conformity procedures applies, select one conformity assessment procedure that has been applied: the verification procedures, the production quality assurance procedures; the product quality assurance procedures> have also been applied to the device. Each kind of medical device to which the technical documentation applies complies with the applicable provisions of the essential principles, the classification rules, and these procedures.
For All Class I
Each kind of medical device to which the technical documentation applies complies with the applicable provisions of the essential principles and the classification rules before being supplied.
For Class IIa, Class I sterile, & Class I Measurement medical devices; and for Class 1 and Class 2 IVD’s choose ONE conformity assessment procedure applied to the device and include the certificate number on this declaration
Certificate number for the verification procedures
This may include:
TGA issued conformity assessment certificate(s)- Schedule 3, Part 3- verification procedures; OR
MRA conformity assessment body certificate(s); OR
European conformity assessment certificate under Annex IV of the Directive 93/42/EEC on Medical Devices; OR
See attached schedule for multiple certificates>
OR
Certificate number for the production quality assurance procedures
This may include:
TGA issued conformity assessment certificate(s) – Schedule 3, Part 4- production quality assurance procedures; OR
MRA conformity assessment body certificate(s); OR
European conformity assessment certificate under Annex V of the Directive 93/42/EEC on Medical Devices; OR
See attached schedule for multiple certificates>
OR
Certificate number for the product quality assurance procedures:
This may include:
TGA issued conformity assessment certificate(s) – Schedule 3, Part 5- product quality assurance procedures; OR
MRA conformity assessment body certificate(s); OR
European conformity assessment certificate under Annex VI of the Directive 93/42/EEC on Medical Devices; OR
See attached schedule for multiple certificates
Standards applied: Please give details of any conformity assessment standard or medical device standard that has been applied to the kind of medical device; OR
See attached schedule for multiple standards
Authorised signatory: signed by the person authorised by the manufacturer
Authorised signatory:
Signature
Name, Position Date




