Table of Contents
When to implement UDI? For CE

Eventually all Medical Device entering EU markets under than custom made and performance studies/ investigational devices.
Most of the time the question is when should the manufacturer be ready to start the UDI process and not how to go about it.
This article will attempt to de-mystify when to implement UDI?:
- Firstly the timeline is tagged to 3 actions: assignment of UDI, submission of UDI data via Eudamed and lastly implement the UDI on the packaging or on the device itself.
- Then you need to establish the classification of the device.
- And also consider if you still eligible to place your market in the EU Market under MDD (instead of MDR).
- In the scenario that you can go for the MDD route, it does not mean you cannot voluntarily use the MDR route instead!
- If your Medical Device is under the MDD route, UDI requirement does not apply however your notified body (if any) may ask you when is your UDI implementation date.
- Under EU MDR/ IVDR, the UDI assignment date (this is putting the UDI information in applicable documents and EU DOC) is 26 May 2021 for MDR and 26 May 2022 for IVDR
- The obligation to submit UDI data into Eudamed is 26 Nov 2022 for MDR and 26 Nov 2023 for IVDR. However this date is not fixed yet and may be subjected to changes due to the possibility that Eudamed might not be ready by then.
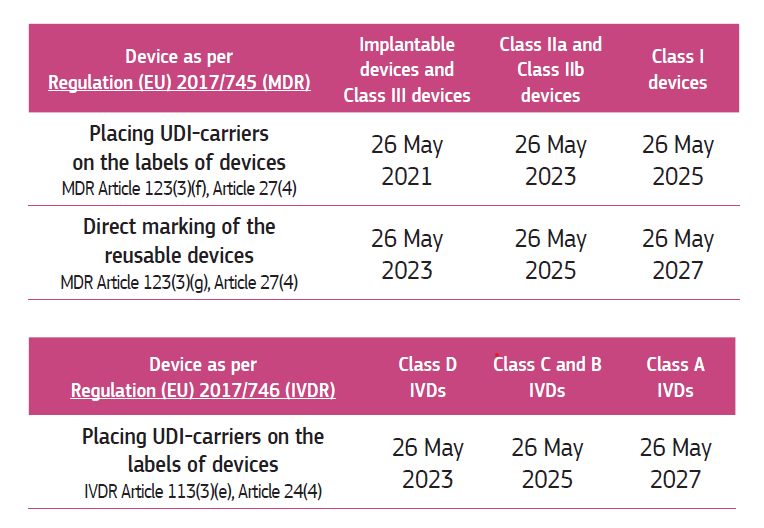
- The obligation to put the UDI carrier on the packaging or device itself is shown on the table below:
When to implement UDI? For Singapore

Updated 29th Oct 2020: The Medical Devices Branch had organised a webinar on the Medical Device Unique Device Identification (UDI) System in Singapore on 19 October 2020 to provide an introduction to Unique Device Identification (UDI) system for medical devices (MD) and the phased implementation plan for Unique Device Identification in Singapore.
Visit here for more information.




