Table of Contents
Free ISO 13485 Training Procedure Template
Free ISO 13485 Training Procedure Template
How to comply
6.2 Human resources
Personnel performing work affecting product quality shall be competent on the basis of appropriate
education, training, skills and experience. By practice this can be defined in Job descriptions.
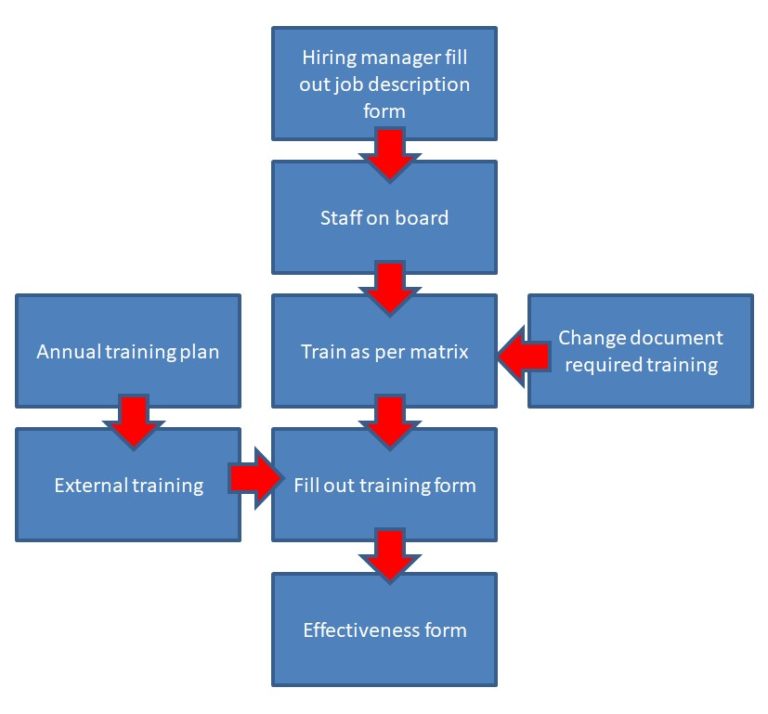
The organization shall document the process(es) for establishing competence, providing needed training, and ensuring awareness of personnel.
Even though an SOP is not needed per say by ISO 13485, most organization have one SOP on this. Besides US 21 CFR 820 requires this as well!
The organization shall:
a) determine the necessary competence for personnel performing work affecting product quality; This means putting up a training matrix for example a production engineer needs to be train in process validation SOP and QA engineer needs to train in Control of Document SOP. It is usually in a from of a table.
b) provide training or take other actions to achieve or maintain the necessary competence; This means train by other staff, own reading to send for external training
c) evaluate the effectiveness of the actions taken; This can be investigated during Non conformance or internal audit. But you can also do a performance review annually. Doing effectiveness check after completion of each training is not very lean.
d) ensure that its personnel are aware of the relevance and importance of their activities and how
they contribute to the achievement of the quality objectives; This can be done during training and link their training to quality objectives: For instance accurate delivery or less production rejects/ or number of non-conformities.
e) maintain appropriate records of education, training, skills and experience have a training record per personnel.
Download Links
Please email info@cmsmedtech.com
Flow Chart